Therma Bright Files Application to the OTCQB and DTC Clearing
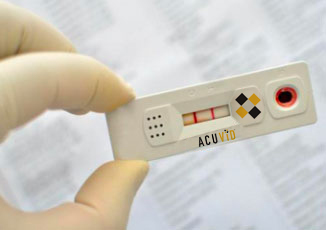
Toronto, Ontario–(Newsfile Corp. – July 29, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to announce that it has applied for OTCQB listing and full eligibility through the Depository Trust Company (DTC), […]
Therma Bright Awaits FDA-EUA Approval From Over 260 AcuVid(TM) Clinical Study Test Results

Toronto, Ontario–(Newsfile Corp. – July 22, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to provide an update on its AcuVid™ COVID-19 Rapid Antigen Saliva Test and awaits an official response from […]
Therma Bright to Test AcuVid(TM) on the Highly Contagious COVID-19 Delta Variant, Company Completes Brazilian Clinical Study for Final FDA EUA Submission

Toronto, Ontario–(Newsfile Corp. – June 24, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to announce that it will test the SARS-CoV-2 (COVID-19) Delta variant, first detected in India, with its AcuVid™ antigen […]
Therma Bright Reports Performance of Its AcuVid(TM) COVID-19 Rapid Antigen Saliva Test from Brazilian Clinical Study

Toronto, Ontario–(Newsfile Corp. – June 17, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of the AcuVid™ COVID-19 Rapid Antigen Saliva Test and a progressive medical device technology company, is pleased to provide an update on the exceptional performance of its AcuVid™ COVID-19 Rapid Antigen Saliva Test’s Brazilian Clinical Study, conducted […]
Therma Bright Provides Update on Brazilian Clinical Study

Company advances final validation efforts of its AcuVid(TM) COVID-19 Rapid Antigen Saliva Test Toronto, Ontario–(Newsfile Corp. – May 27, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to provide the following update on […]
Therma Bright Secures Development and Manufacturing Partner for AcuVid(TM) COVID-19 Rapid Antigen Saliva Test

K-One MediTech To Begin Production of CE approved 15-Minute Antigen Test Solution Toronto, Ontario–(Newsfile Corp. – May 21, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to announce that it has secured a […]
Therma Bright Enters into Agreement to Distribute a 15 Minute Rapid COVID-19 Antibody Test
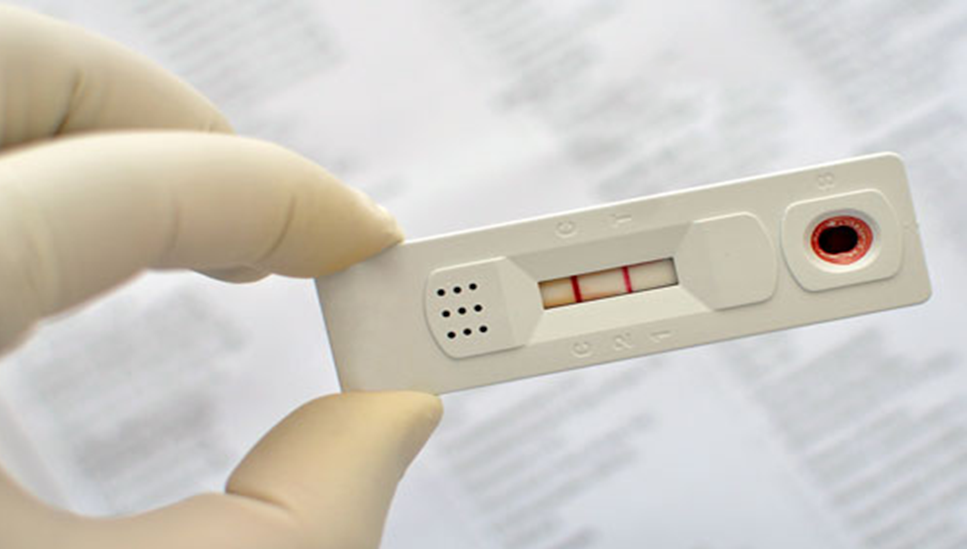
Toronto, Ontario–(Newsfile Corp. – May 6, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of the AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to announce that it has entered into a distribution agreement to white label and distribute a 15-minute rapid COVID-19 […]
Therma Bright Partners With Afero To Make AcuVid(TM) COVID-19 Rapid Antigen Saliva Test Smart-Enabled

Silicon Valley tech firm brings encrypted solution for tracking Canadian developer’s 15-minute Covid19 rapid antigen test for point of care, and then at-home testing Toronto, Ontario–(Newsfile Corp. – April 28, 2021) – Afero has entered into a memorandum of understanding (MOU) agreement with Therma Bright Inc. (TSXV: THRM), the “Company” or “Therma Bright”), developer of […]
Therma Bright’s AcuVid(TM) COVID-19 Rapid Antigen Saliva Test Detects the Important SARS-CoV-2 Variants of Concern; the Brazilian P.1 and P.2 and the UK B.1.1.7 Variants

Toronto, Ontario–(Newsfile Corp. – April 22, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of the AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to announce successful preliminary results with its AcuVid™ COVID-19 Rapid Antigen Saliva Test to detect the SARS-CoV-2 Variants of […]
Therma Bright Announces EU-CE Approval for AcuVid(TM) COVID-19 Rapid Antigen Saliva Test and Conditional Sale for 100,000 CE-only AcuVid(TM) Test Kits

New global distribution partner McWilliams Collective secures test kit order from European client Toronto, Ontario–(Newsfile Corp. – April 19, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of the AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to announce that it has received CE […]