
**This product is not currently ready for sale**
AcuVid™ aim is to make the world a safer place and allow economies to open and stay open by offering simple, low-cost antigen and antibody rapid screening test for COVID-19.
The World Health Organization sent a clear message to all countries in March 2021: test, test, and test… The more tests that are conducted, the easier it becomes to track the spread of the virus and reduce transmission.
**This product is not currently ready for sale**
COVID-19 Rapid Saliva Antigen Test
AcuVid™ COVID-19 Rapid Antigen Saliva Test aim is to make the world a safer place, allowing economies to re-open safely and stay open, while mitigating the spread of COVID-19 by infected and asymptomatic people. AcuVid™ Covid-19 Tests offer a simple, low-cost rapid screening solution for the immediate detection of the novel coronavirus (SAR-CoV-2) Wuhan strain, as well as other COVID-19 variants, such as the Brazilian P.1 and P.2 strain and the UK B.1.1.7 strain.
In March 2021, both the World Health Organization (WHO) and the US Centers for Disease Control (CDC) sent a clear message and guidance for all countries to conduct serial testing. Serial testing involves testing the same individual multiple times within a few days, and can increase chances of detecting asymptomatic infection that might not always show up with a single test. The more tests that are conducted, the easier it becomes to track the spread of the virus and reduce transmission.
The AcuVid™ Covid-19 Rapid Antigen Saliva Test is very simple to use:
Point-of-Care/Health Professional Testing: Our initial use of the AcuVid™ COVID-19 Rapid Antigen Saliva Test will be at point-of-care (PoC) facilities and/or with health care professionals who may be stationed in public spaces to test the general public, employees or customers as required by the places they visit.
Use at Health Care Facilities or places with Health Care Practitioner
At-Home / On-The-Go Testing: Once approved for At-Home & On-The-Go Testing, our smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test can then be purchased online or via over-the-counter at your local grocer or pharmacy for testing in the privacy of your own home.
At-Home & On-The-Go-Testing places:

Our partners, nanoComposix, out of California, USA, is a leading custom developer of rapid ultra- sensitive lateral flow tests. The company has over 14 years of customized cGMP manufacturing capabilities, is ISO-13485 certified and recognized as a World-leading manufacturer of precisely engineered and highly characterized nano materials. They produce the most critical part of the AcuVid™ COVID-19 Rapid Antigen Saliva Test, which is the lateral flow strip that can be found in the test kits cartridge and using your saliva and a reagent liquid, detects the novel coronavirus (SAR-CoV-2) Wuhan strain, as well as variants, including Brazilian P.1 and P.2 and the UK B.1.1.7 strains.
Initial AcuVid™ COVID-19 Rapid Antigen Saliva Tests included the use of frozen saliva specimens. Additional live human saliva tests were then conducted in Brazil at the Federal University of Minas Gerais (UFMG).
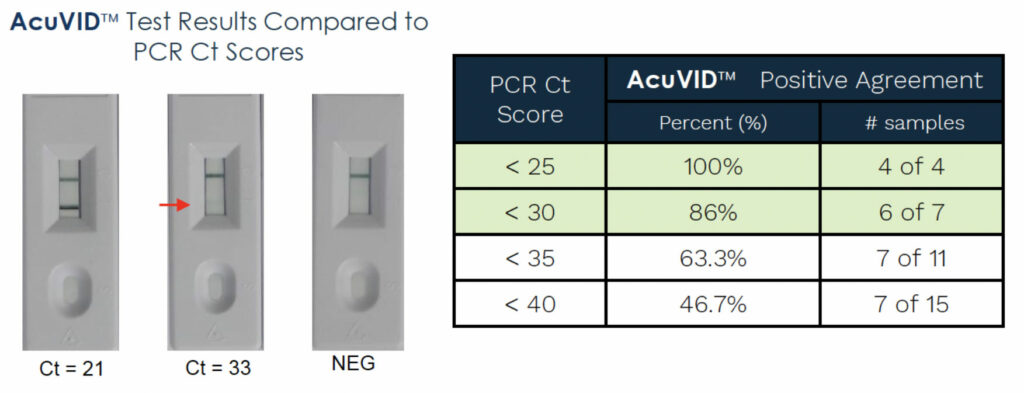
In addition to the successfully testing or our COVID-19 Rapid Antigen Saliva Test with the original Wuhan strain of the novel coronavirus (SAR-CoV-2), our Brazilian partners successfully detected other COVID-19 variants, such as the Brazilian P.1 and P.2 strain and the UK B.1.1.7 strain.
Therma Bright’s white-labeled AcuVid™ COVID-19 Rapid Antibody Test is a simple pinprick antibody blood test (i.e. serology test) that uses a small amount of blood. The antibody test has a 96.6% sensitivity for detecting antibodies of SARS-CoV-2 in those individuals currently infected with the virus or who have previously been infected, but went undiagnosed or were unaware of their infection. It will also aid in determining antibodies generated by those who have received a COVID-19 vaccine.
This new white labeled AcuVid™ COVID-19 Rapid Antibody Test offers users a simple 15-minute rapid COVID-19 antibody test for detecting IgG and IgM antibodies against SARS-CoV-2 (the original Wuhan novel coronavirus) and its variants.
Using a small droplet of blood and a buffer solution is the first step in this simple test procedure. The next step is applying the both the droplet of blood and buffer onto the lateral flow test cartridge for the detection and presence of both the IgG and IgM antibodies. Wait 15 minutes to view results.
Studies have shown the importance of antibody testing, along with serial antigen testing, in controlling and monitoring COVID-19 and vaccine effectiveness. In March 2021, FDA Guidance was provided to test developers who were seeking emergency use authorization (EUA) of certain tests that could be used for serial testing, which includes the AcuVid™ COVID-19 Rapid Antigen Saliva Test. Serial testing involves testing the same individual multiple times within a few days, and can increase chances of detecting asymptomatic infection that might not always show up with a single test. CDC recommends serial testing at least once per week, along with other mitigation measures, such as masking and social distancing, to reduce disease transmission. This AcuVid™ Antibody test will be Therma Bright’s second rapid test for serial screening.
Therma Bright Inc.
345 Danforth Avenue
Toronto, ON M4K 1N7