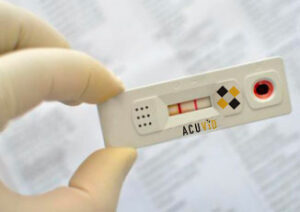
Toronto, Ontario–(Newsfile Corp. – June 24, 2021) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of its smart-enabled AcuVid™ COVID-19 Rapid Antigen Saliva Test and other progressive diagnostic and medical device technologies, is pleased to announce that it will test the SARS-CoV-2 (COVID-19) Delta variant, first detected in India, with its AcuVid™ antigen saliva test. Governments and global health experts have expressed deep concerns that the highly contagious, fast spreading Delta variant will add to a new wave of COVID-19 viral infections globally later this summer and fall. The Delta variant has already become the dominant version of the SARS-CoV-2 (COVID-19) virus in the United Kingdom and has been detected in at least 74 other countries worldwide,
In addition, the Company has received the final 7 outstanding RT-PCR test results from the Brazilian study to match against its AcuVid™ saliva test, exceeding the minimum requirements of 30 positive/30 negative results for its FDA Emergency Use Authorization (EUA) submission. The study results will also be used for ANVISA (Brazil) and INVIMA (Colombia) submissions, as well as to further support the CE approval received in April 2021.
“We are excited that our Brazilian clinical study has now met the minimum FDA-EUA requirements for our submission to secure serial testing approval,” expressed Rob Fia, Therma Bright’s CEO. “In addition, we’re equally excited to begin testing our AcuVid™ COVID-19 Rapid Antigen Saliva Test with the Delta variant; as we have successfully tested other fast-moving SARS-CoV-2 variants, including the P.1 and P.2 variants originally detected in Brazil and the B1.1.17 variant first detected in the UK. As always, our goal is to make our 15-minute AcuVid™ COVID-19 antigen test the leading rapid screening solution to help mitigate community spread of this virus.”
Therma Bright is not making any express or implied claims that its test product has the ability to eliminate or cure COVID-19 or the SARS-CoV-2 virus.