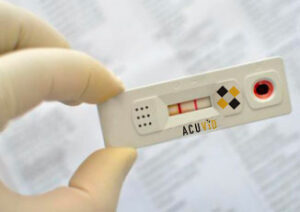
Toronto, Ontario–(Newsfile Corp. – September 3, 2020) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), a progressive medical device technology company, is pleased to announce that its co-development partner, Orpheus Medica Inc. (“Orpheus”) has identified a series of polypeptides for the screening and detection of the virus (SARS-CoV-2) causing COVID-19.
The research carried out by Orpheus and its academic collaborators has resulted in several high-quality polypeptides that recognize the COVID-19 virus with clear distinction from other coronavirus types such as SARS or MERS. These biological molecules will form the basis of the CoviSafe™ that is being developed to screen and detect the proteins of the virus rather than its genetic material. Therma and Orpheus expect to provide additional test formats where the user can test and differentiate between COVID-19 and seasonal flu symptoms.
Recently, Health Canada announced that it will be taking applications for home testing devices for screening purposes for COVID-19.
See National Post: https://nationalpost.com/pmn/health-pmn/health-canada-changes-course-on-covid-testing-at-home
CoviSafe™ is a screening test designed to monitor large populations of healthy individuals that are asymptomatic for the COVID-19 illness. CoviSafe™ can also be used as a diagnostic test to investigate individuals with COVID-like symptoms. CoviSafe™ will have a number of quality control features to avoid misuse or misinterpretation of home-based test results, which address a number of Health Canada’s concerns as outlined in the article by the National Post. Therma and Orpheus will be carrying out field evaluation testing to determine test performance in different settings. The goal is to provide our solution for routine and widespread testing at schools, workplaces, sporting events or at home.
Dr. Saeid Babaei, Chairman & CEO of Orpheus commented: “We are excited to identify and validate the first series of our polypeptides in collaboration with our academic partners in a timely fashion. The detection molecules discovered to-date have already been validated in a COVID-19 model in the laboratory. Our plan is to further validate these results on the device platform as the next step in coming weeks.”
Mr. Rob Fia, CEO of Therma Bright, commented: “We believe that CoviSafe™ can be developed as a low-cost, saliva-based solution, that produces fast and accurate results for use at point of care or home, school, business or sporting event applications. We are excited for the potential to incorporate features that identify whether someone has COVID-19 or the seasonal flu. Therma and Orpheus believe this goal is the game-changer that the world is looking for to get back to normalcy.”