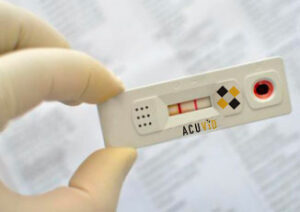
Smart-enabled AcuVid™ Antigen Test
On April 28, 2021, Therma Bright announced a memorandum of understanding (MOU) agreement to incorporate a secure tracking technology into its premier AcuVid COVID-19 15-Minute Rapid Antigen Saliva Test. (See Press & Media Section). The smart-enabled antigen test is geared to assist communities in safely and securely return to work and play, while using an embedded and encrypted technology for tracking and reporting Covid-19 test results. The smart solution will not only assist in tracking product for quality assurance, shipping and logistics, but will also be used at point-of-care for reporting test results. The smart-enabled solution also paves the way for Therma Bright to prepare its AcuVID COVID-19 Rapid Antigen Test for at-home testing governmental approval and personal use. Overall, this smart-enabled AcuVid™ test will track and help individuals manage their results on a real-time basis for the millions of tests the Company plans to deploy around the world. And with this excrypted technology, millions of test results can then be shared safely and securely with government agencies, workplaces, entertainment venues and more. Rob Fia, CEO of Therma Bright, believe that their premier, smart-enabled AcuVid COVID-19 Rapid Antigen Test will provide a “Brighter Experience” a complete end-to-end solution that ensures product tracking, quality control and test validation data are all cryptographically captured and secured to protect individual privacy with every test taken.
The opportunity of working with such lead-edge technology will allow Therma Bright to further help in mitigating community spread of the original COVID-19 virus (Wuhan SARS-CoV-2), as well as growing list of variants, including the Brazilian P.1 and P.2 and the UK B.1.1.7 variants. Three variants which the Company has successfully detected in their research with the Federal University of Minas Gerais (UFMG) in Brazil. All in all, things are looking better and brighter each day with Therma Bright and its ongoing pursuit to bring its innovative COVID19 diagnostic test to the global marketplace.