Products
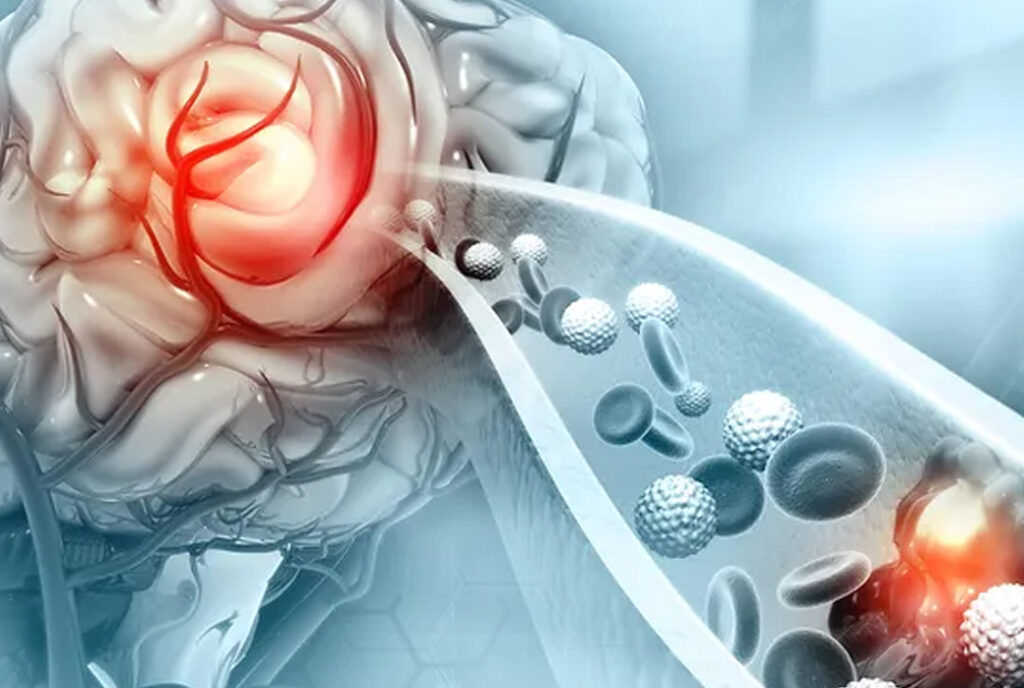
Therma Bright and Inretio are revolutionizing ischemic stroke treatment with their innovative clot-retrieving device, PREVA™. Ischemic strokes are caused by a clot blocking blood flow to the brain and afflict over 690,000 US patients each year. Current clot-retrieving devices offer incomplete solutions, whereas Inretio’s technology aims to improve effectiveness and prevent complications during thrombectomy procedures. This breakthrough has the potential to significantly impact the lives of those affected by ischemic strokes and make a substantial difference in the medical community.
The PREVA™ clot retriever offers a new approach to clot retrieval by reaching distally to the clot and opening a patented protective basket. This method minimizes the risk of embolization and reduces the need for repeated maneuvers in the procedure. The device’s unique PREVA™ basket “ensnares” the clot, encapsulating it and protecting the brain from sub-clots breaking off during the thrombectomy. This complete removal of the clot and its fragments leads to more successful revascularization, preventing further damage and complications.
The PREVA™ device has shown promising results in early animal trials, achieving an 80% success rate compared to the 50% effectiveness of existing clot retriever technology. Inretio has received funding from the Israel Innovation Authority for full development of the technology and a working prototype. Plans for 2023 include additional animal studies until March 2023 and clinical trials by July 2023 at locations worldwide, including Israel, Canada, and the US at renowned hospitals.
The global market for clot retrievers, currently dominated by major US medical device companies, is valued at $2.83 billion in 2022 and expected to grow.
PREVA works by using a unique approach to clot retrieval in ischemic stroke treatment. The device features a patented protective basket that opens distally to the clot. During the thrombectomy procedure, the PREVA basket “ensnares” the clot, encapsulating it and preventing any sub-clots or fragments from breaking off and causing further damage. This method minimizes the risk of embolization and reduces the need for repeated maneuvers in the procedure. By completely removing the clot and its fragments, PREVA enables more successful revascularization of the brain, which can prevent further damage and complications for stroke patients.
Press Releases
Therma Bright Provides Update on FDA EUA Application Process For AcuVid(TM) COVID-19 Rapid Antigen Saliva Test
Toronto, Ontario–(Newsfile Corp. – June 2, 2022) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of the smart-enabled AcuVid™ COVID-19 Rapid Antigen

Therma Bright to Submit Application to Health Canada for Approval of its AcuVid(TM) COVID-19 Rapid Antigen Saliva Test
Toronto, Ontario–(Newsfile Corp. – May 10, 2022) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), a progressive medical device technology company, is pleased

Therma Bright Submits AcuVid(TM) COVID-19 Rapid Antigen Saliva Test Application for U.S. Food & Drug Administration’s Emergency Use Authorization (EUA)
V.THRM | 1 minute agoToronto, Ontario–(Newsfile Corp. – March 29, 2022) – Therma Bright Inc. (TSXV: THRM) (OTCQB: TBRIF) (“Therma” or the “Company”), developer of its