Products

The Digital Cough Test (DCT) is an app created by Therma Bright and AI4LYF that uses artificial intelligence to detect multiple respiratory diseases, including COVID-19, by analyzing a person’s cough sound. With an accuracy above 94%, DCT provides rapid results comparable to PCR tests and superior to Lateral Flow Kits. The technology is non-invasive, cost-effective, environmentally friendly, and easily repeatable.
Users simply install the DCT app, record a 3-second cough sample, and receive results in less than 30 seconds. The app is compatible with any mobile phone and internet connection.
AI4LYF’s STOPP solution, a new outbreak monitoring system, aims to predict and prevent future pandemics when deployed at scale. DCT has various use cases, including governance during pandemics, healthcare, education, travel, and work.
The AI algorithm learns to identify diseases by analyzing cough sounds from thousands of samples. It detects unique sound attributes and frequencies that the human ear cannot discern, resulting in accurate diagnoses.
The Digital Cough Test (DCT) works by using artificial intelligence to analyze a person’s cough sound, identifying unique sound attributes and frequencies associated with respiratory diseases like COVID-19. The AI algorithm is trained with thousands of cough samples, enabling it to discern subtle patterns in the cough sounds and provide an accurate diagnosis that is difficult for the human ear to detect.
Press Releases
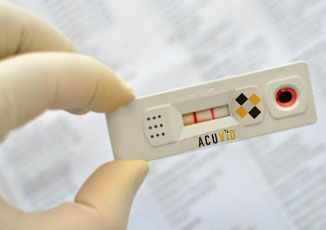
Therma Bright Provides Update on FDA EUA Application Process For AcuVid(TM) COVID-19 Rapid Antigen Saliva Test
Toronto, Ontario–(Newsfile Corp. – June 2, 2022) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), developer of the smart-enabled AcuVid™ COVID-19 Rapid Antigen

Therma Bright to Submit Application to Health Canada for Approval of its AcuVid(TM) COVID-19 Rapid Antigen Saliva Test
Toronto, Ontario–(Newsfile Corp. – May 10, 2022) – Therma Bright Inc. (TSXV: THRM) (“Therma” or the “Company”), a progressive medical device technology company, is pleased

Therma Bright Submits AcuVid(TM) COVID-19 Rapid Antigen Saliva Test Application for U.S. Food & Drug Administration’s Emergency Use Authorization (EUA)
V.THRM | 1 minute agoToronto, Ontario–(Newsfile Corp. – March 29, 2022) – Therma Bright Inc. (TSXV: THRM) (OTCQB: TBRIF) (“Therma” or the “Company”), developer of its